Scientists responsible for developing what they describe as a hugely promising COVID-19 vaccine candidate say they can complete human trials and produce 30 million doses by January 2021 on less than a single day’s worth of Macau 2019 casino revenue.
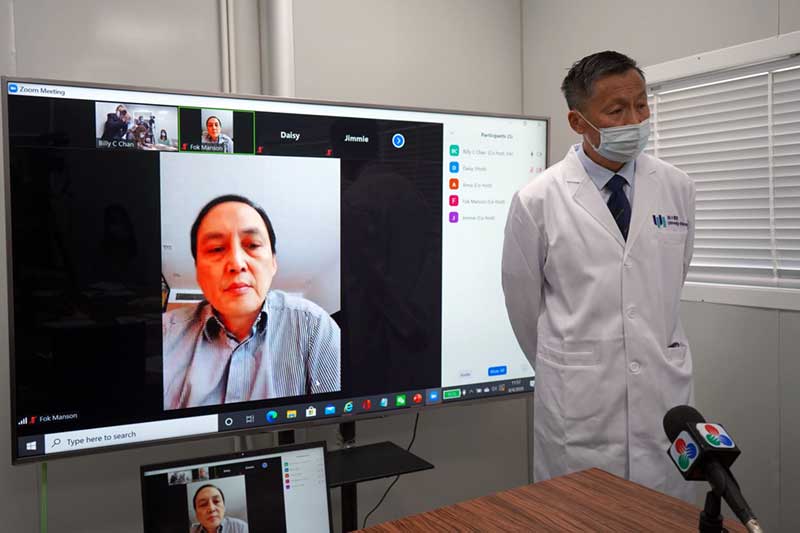
The revelations came at a press conference at the Faculty of Medicine at Macau University of Science and Technology (MUST) on Tuesday. The press conference was led by Professor Zhang Kang in Macau, and Professor Manson Fok, Dean of the Faculty, who appeared from Hong Kong by video.
The vaccine, announced last week by a team comprising researchers from MUST, in collaboration with Hong Kong Polytechnic University (PolyU), is seen as a leading candidate for mass production after what the researchers describe as exceptional results during early clinical trials. In particular, it induced a “potent functional antibody response” in immunized mice, rabbits and monkeys with those antibodies subsequently neutralizing the virus during laboratory studies by effectively blocking it from binding with cells in the host.
According to key scientists involved, the candidate vaccine is now ready to enter Phase II and Phase III trials which, if all goes well, could see the first 30 million doses manufactured and ready for distribution by late January.
However, at the press conference, it was revealed the project was at risk of stalling over a shortfall in funding. Speaking of the world’s largest casino hub, Professor Fok estimated HK$600 million (US$77 million), equivalent to 77% of one day of Macau casino revenue based on 2019 GGR, or just two days’ worth of gaming taxes collected by the Macau SAR Government, would be needed to develop and produce 30 million doses of the vaccine.

The team revealed it had been knocked back in February after applying to the Macau government for funding of just MOP$500,000 (US$62,500), with Professor Fok indicating the government had not been convinced at the time that development of a vaccine was a realistic goal.
According to the MUST team, the cost of the collaborative research, which has involved more than 100 scientists from Macau, Hong Kong and mainland China, is already in the “tens of millions of US dollars” – all of it sourced from the internal resources of eight institutions involved.
But the team is also adamant their vaccine ranks among the most effective, perhaps even the most effective, of the more than 150 vaccine candidates currently being studied globally.
“From a conceptual standpoint, we think this will be a highly effective vaccine with a very low level of side effects,” said Professor Zhang, one of the team’s leaders.
According to Zhang, the team’s vaccine stands out from most others because it only attacks the Receptor Binding Domain (RBD) of the protein rather than the whole protein spike, therefore creating more effective antibodies.
The vaccine is also a standout because it utilizes recombinant DNA, such as that already used in the HPV vaccine widely used to combat various forms of cancer and genital warts. The technology is already readily available and used in manufacturing facilities around the world.
Zhang said the team initiated development of its vaccine shortly after Chinese New Year, with Phase I testing showing no side effects. They are also encouraged by the fact that the vaccine requires administration of an extremely small dose – around 20 micrograms or 1/10th the size of a sesame seed.
Notably, Zhang says the vaccine should even be able to treat those already sick with COVID-19.
The team is now waiting to progress to the next step, Zhang said, where it plans to run Phase II and Phase III trials in humans concurrently to expedite the process.
“We are very confident from our results in the animal studies of its worth,” he said.
Fok added, “Normally human trials to deployment is one-and-a-half to two years,” but pointed to the fact that COVID-19 is not a normal situation. “So far the vaccine has shown strong antibody response, no complications, a significant half-life and it will be very cost effective.”

Fok did, however, note the vaccine could not be produced locally due to the absence of a vaccine facility in Macau, and a lack of testing capacity in mainland China due to a number of other candidate vaccines being tested. Instead, the MUST team has agreed to conduct a pilot manufacturing program with a partner in Taiwan, which they say has the capability to produce at least 30 million doses in a matter of weeks.
As previously reported by IAG, 8 million of those would be reserved for the people of Macau and Hong Kong. The team, Fok claimed, wants to “produce a vaccine for all of humanity.
“This is a global problem and needs a global solution,” he said.



































